Choosing A Suitable Endotoxin Test
Pharmaceuticals that are contaminated with endotoxins lead to pyrogenic responses. Stringent microbiological quality control assessments apply to all medicines and medical equipment described as being ‘sterile products’ to verify adherence to manufacturing and product release specifications.
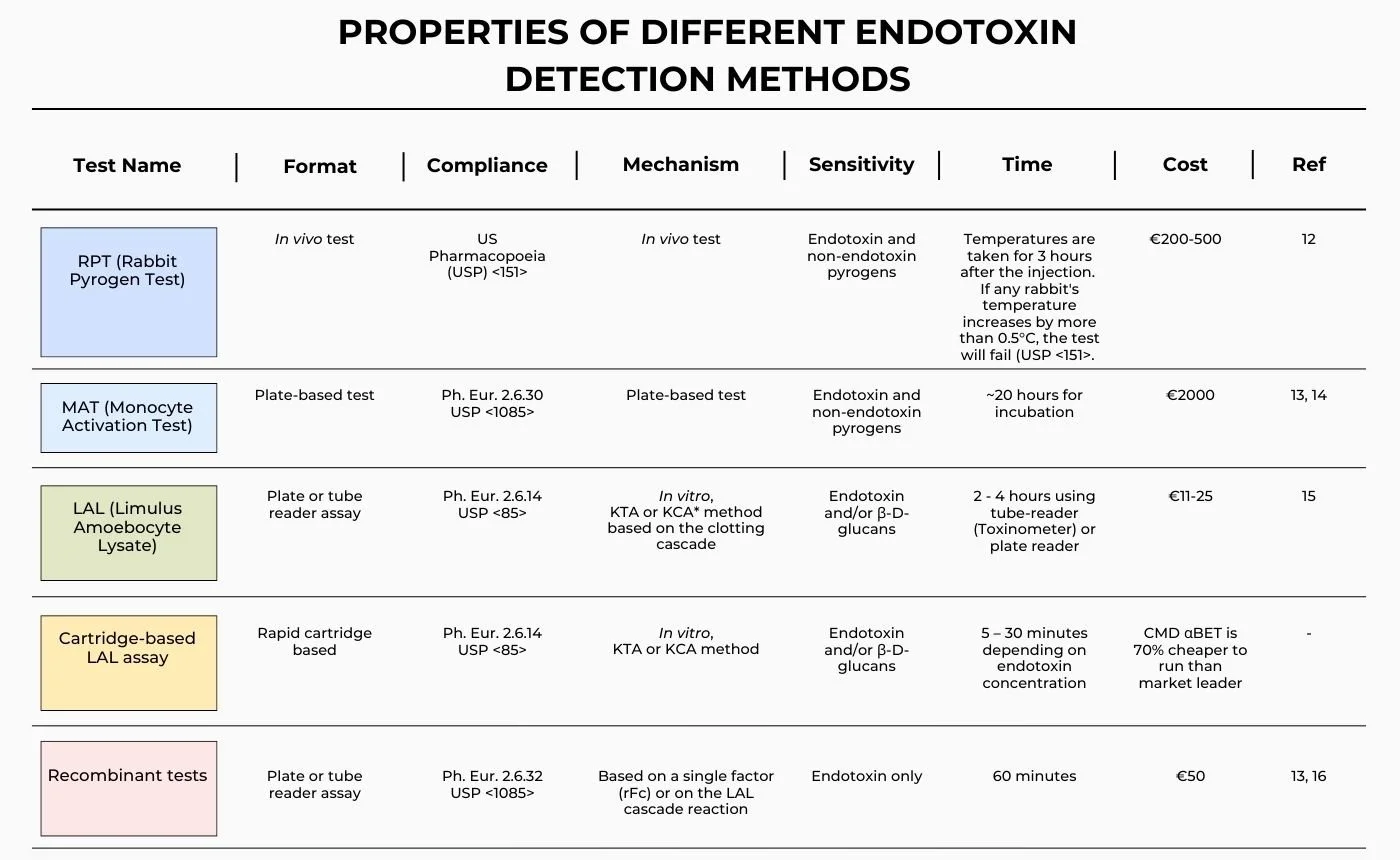
Different Endotoxin Tests
The presence of pyrogens in sterile products, endotoxin being the most important, poses a significant risk to human health. Historically, ‘pyrogenicity’ has been determined using the Rabbit Pyrogen Test (RPT), increasingly being replaced by the Monocyte Activation Test (MAT), whereas endotoxin is more specifically tested for using the Limulus Amebocyte Lysate (LAL) assay and, recently, by recombinant versions of the LAL assay. Declarations of regulatory compliance rely on testing being performed as described in the United States, British, European and Japanese Pharmacopoeias [1].
LAL (Limulus Amoebocyte Lysate) and Rabbit Pyrogen Test (RPT)
The rabbit pyrogen test, developed in 1912, uses rabbits to detect pyrogenic substances by monitoring the rabbit's body temperature for febrile reaction to injected medicines. However, the response of rabbits may differ from that of humans, which may lead to misleading results [2]. Whilst there is a move away from the use of rabbits, the RPT is still used for certain products, including vaccines, blood products and antibiotics. The LAL test was approved for use by the FDA in 1977, and subsequently introduced into other pharmacopoeias around the world. This assay now dominates the endotoxin testing market, being valued for its cost-effectiveness when compared to the RPT, and sensitivity to endotoxin [3]. It has been shown to be five times more sensitive than the RPT for pyrogen detection [4].
However, the LAL test also has limitations, both in its nature and performance. Whilst it does not use materials derived from mammals (e.g., rabbits), it is based on a reagent derived from the North Atlantic Horseshoe Crab and therefore cannot be considered an ‘animal-free’ product. In the context of pyrogen testing, there are also regulatory considerations. Endotoxin is the most potent pyrogenic substance known, and regulators base pyrogenic risk wholly on the level of endotoxin detected in a product. In fact, endotoxin is the only pyrogen for which regulatory binding limits exist. However, many LAL-based endotoxin assays also respond to other pyrogens, such as (1-3) β-D-glucan, which potentially can give rise to false-positive endotoxin tests [5]. Endotoxin-specific LAL-reagents do exist, and one is available from FUJIFILM Wako (PYROSTAR ES-F™). This reagent provides end-users with increased confidence that product failures are genuine endotoxin failures. More significantly, from a safety perspective, some products contain factors that interfere with the LAL assay and can result in dangerous false-negative endotoxin results. For instance, the LAL reaction requires divalent cations to proceed and so, in the absence of appropriate controls and informed method development, the presence of materials that chelate these ions can result in dangerous false negative results. Similarly, products that mask the presence of endotoxin (such as biopharmaceuticals that commonly contain high concentrations of biomacromolecules) can also give rise to a false negative endotoxin test [6]. Whilst being relatively straightforward to perform and quite cost-effective, the LAL assay is susceptible to a range of product interferences, and method development should always be performed by an experienced team.
In June 2024, the European Pharmacopoeia (Ph. Eur.) decided to remove the RPT from its monographs and adopted 57 revised texts, from which the RPT had been deleted, and a new general chapter Pyrogenicity (5.1.13). Pyrogen testing guidance will ask the medicine developer to select a suitable in vitro test. It is likely that the natural replacement for the RPT will be the MAT assay [7,8]. The revised texts were published in Supplement 11.8 of Ph. Eur. and implemented in July 2025. Currently, this move away from RPT has not been adopted in the USP.
MAT (Monocyte Activation Test)
By employing human cells, the MAT test aims to have improved relevance to a mammalian whole-body febrile response analogous in many ways to the rabbit pyrogen test. The MAT assay is not specific to endotoxin but provides a semi-quantitative measure of cellular response to the presence of pyrogens [9]. To comply with FDA guidelines, it is essential to perform product-specific validation to assess the suitability of using the MAT technique for evaluating the specific test substance or material [10].
Substantial research and effort have demonstrated MAT is a suitable RPT alternative. An international conference titled "The future of pyrogenicity testing: phasing out the rabbit pyrogen test" was jointly hosted by the European Directorate for the Quality of Medicines & HealthCare, the Council of Europe, and the European Partnership for Alternative Approaches to Animal Testing. The conference focused on demonstrating the European Pharmacopoeia's plan to eliminate the RPT from its texts by 2026, streamline the adoption of MAT, and identify any deficiencies in the transition from RPT [9].
Assays based on recombinant LAL proteins
The newest endotoxin reagents to achieve regulatory approval use recombinant proteins from the LAL cascade. Two approaches have been taken: the first uses a recombinant form of Factor C (rFC), the endotoxin binding protein in the LAL cascade; whilst the other approach has been to produce a recombinant version of the complete protein cascade (Factor C, B, and the pro-clotting enzyme) [11]. Recombinant Cascade Reagents (rCRs) have very recently entered the market, providing an animal-free alternative to LAL but with mechanistic continuity with traditional LAL reagents and with greater sensitivity and robustness than rFC reagents. PYROSTAR™ Neo+, a new rCR-based reagent, is presented as a comprehensive and improved solution for endotoxin detection, offering a kinetic chromogenic assay, reduced interference, and increased sensitivity. Due to the cascade reaction, substantial signal amplification is achieved, beyond what is possible with single-factor recombinant reagents such as Factor C..
*KTA = Kinetic Turbidimetric Assay; KCA = Kinetic Chromogenic Assay
References:
Perdomo-Morales, R., Pardo-Ruiz, Z., Spreitzer, I., Lagarto, A. & Montag, T. (2011). Monocyte activation test (MAT) reliably detects pyrogens in parenteral formulations of human serum albumin. Altex, 28(3): 227-235.
Hartung, T. (2015). The human whole blood pyrogen test – lessons learned in twenty years. ALTEX - Alternatives to animal experimentation, 32(2): 79-100.
Mehmood, Y. (2019). What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in Endotoxin Quantification of Pharma Products. In: M. Mishra, Growing and Handling of Bacterial Cultures. IntechOpen. doi:10.5772/intechopen.81331.
Karesh, S.M. (1989). Sterility and pyrogen testing of radiopharmaceuticals. Journal of Nuclear Medicine Technology, 17(3): 156-159.
Piehler, M., Roeder, R., Blessing, S. & Reich, J. (2020). Comparison of LAL and rFC Assays-Participation in a Proficiency Test Program between 2014 and 2019. Microorganisms, 8(3): 418.
Mitra, A., Joshi, S., Arjun, C., Kulkarni, S. & Rajan, R. (2014). Limulus amebocyte lysate testing: adapting it for determination of bacterial endotoxin in 99mTc-labeled radiopharmaceuticals at a hospital radiopharmacy. J Nucl Med Technol, 42(4): 278-282.
EDQM (2024). Ph. Eur. bids adieu to rabbit pyrogen test in its monographs. Available at https://www.edqm.eu/en/-/ph.-eur.-bids-adieu-to-rabbit-pyrogen-test-in-its-monographs
Vipond, C., Findlay, L., Feavers, I. & Care, R. (2016). Limitations of the rabbit pyrogen test for assessing meningococcal OMV based vaccines. Altex, 33(1): 47-53.
Carson, D., Myhill, S., Palmieri, E., Necchi, F., Rijpkema, S., Micoli, F., et al. (2021), Development of a Monocyte Activation Test as an Alternative to the Rabbit Pyrogen Test for Mono- and Multi-Component Shigella GMMA-Based Vaccines. Microorganisms, 9(7): 1375.
Sandle, T. (2013). FDA guidance on pyrogens and endotoxin testing. GMP Review, 12(1): 7-9.
USP (no date). Use of recombinant animal-free reagents in the bacterial endotoxins test. Available at https://www.usp.org/covid-19/treatment-and-prevention/rfc-summary
PW Consulting (2025). Pyrogenicity Testing Service Market. Available at https://pmarketresearch.com/it/pyrogenicity-testing-service-market/
Cirefice, G., Schütte, K., Spreitzer, I., Charton, E., Shaid, S., Viviani, L., et al. (2023). The future of pyrogenicity testing: Phasing out the rabbit pyrogen test. A meeting report. Biologicals, 84: 101702.
Solati, S., Zhang, T. & Timman, S. (2022). The monocyte activation test detects potentiated cytokine release resulting from the synergistic effect of endotoxin and non-endotoxin pyrogens. Innate Immun, 28(3-4): 130-137.
Uchida, T., Kaku, Y., Hayasaka, H., Kofuji, M., Momose, N., Miyazawa, H., et al. (2019). Utility Of An Automatic Limulus Amebocyte Lysate Kinetic Turbidimetric Test For Endotoxin Screening Of Dialysate Samples. Med Devices (Auckl), 12: 429-433.
Marius, M., Vacher, F. & Bonnevay, T. (2020). Comparison of bacterial endotoxin testing methods in purified pharmaceutical water matrices. Biologicals, 67: 49-55.