Does pyrogen-free mean endotoxin-free?
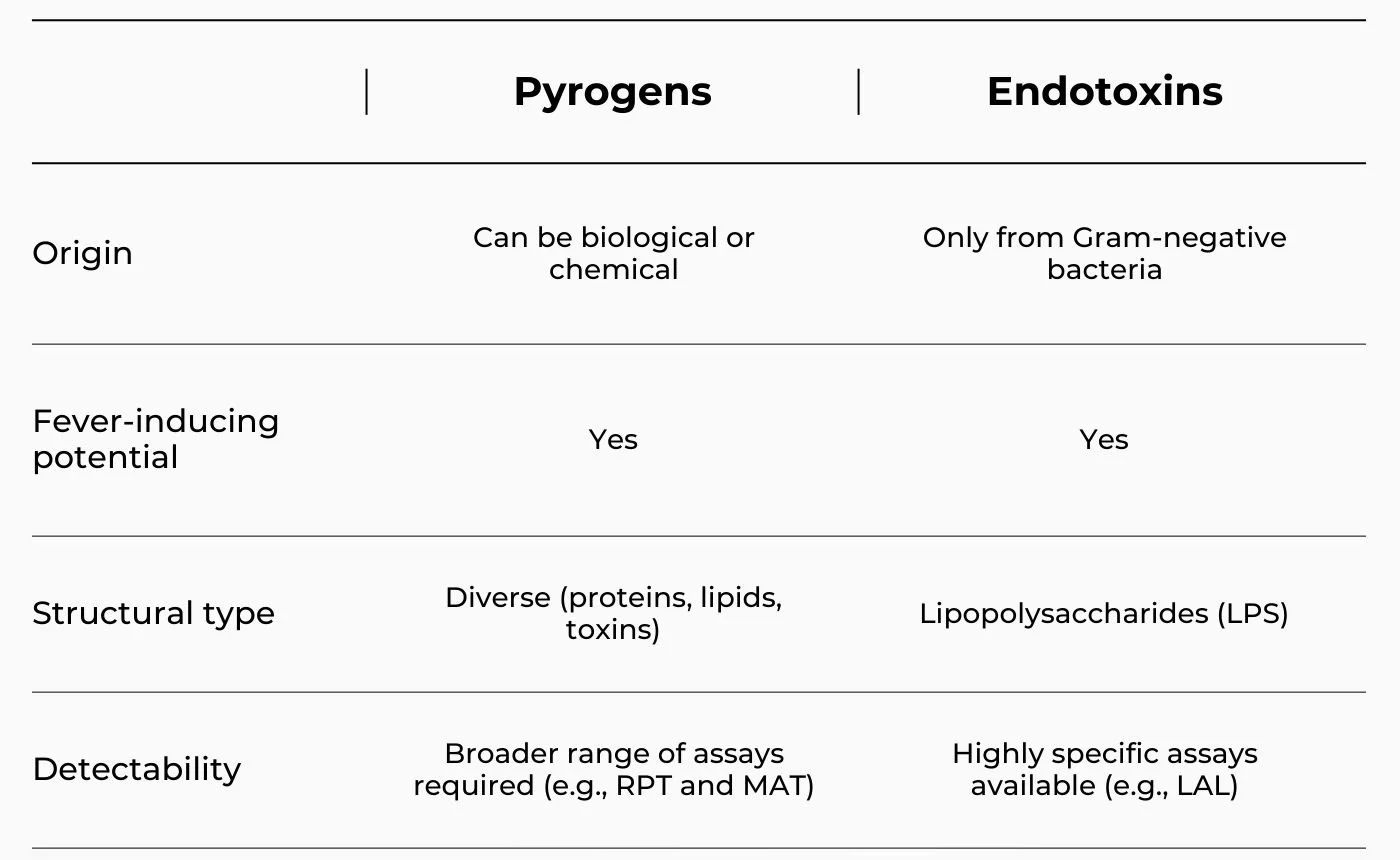
Pyrogens are substances that can induce fever when introduced into the body, whereas endotoxins are a specific type of pyrogen derived from the outer membrane of gram-negative bacteria [1,2]. All endotoxins are pyrogens, but not all pyrogens are endotoxins.
The distinction matters greatly for pharmaceutical manufacturers, medical device producers, and research laboratories since a product can be labelled “pyrogen-free” while still containing non-endotoxin pyrogens, such as microbial fragments or certain chemicals, which may still trigger adverse immune responses [3]. Failure to recognise this distinction can compromise patient safety and regulatory compliance.
Differences Between Pyrogens and Endotoxins
What Each One Is
Pyrogens are fever-inducing substances that stimulate the immune system to release cytokines, leading to increased body temperature. These can originate from microbial contamination, environmental exposure, or even processing residues [4].
Endotoxins are lipopolysaccharide (LPS) components located in the cell walls of Gram-negative bacteria, released when these bacteria grow or die [5].
Sources
Common sources of endotoxins include [1]:
Water systems used in pharmaceutical manufacturing
Raw biological materials
Bacterial contamination during processing
Common sources of non-endotoxin pyrogens include [3,6]:
Gram-positive bacteria
Fungal cell wall components (e.g., β-glucans)
Viral particles
Chemical residues from manufacturing
Examples
Endotoxins:
Lipopolysaccharide (LPS) from Escherichia coli
Non-endotoxin pyrogens:
Lipoteichoic acid from Gram-positive bacteria
Fungal mannans
Mycoplasma membrane components
Are the Testing Methods Different for Pyrogens and Endotoxins?
Yes, the test methods are different. Endotoxins are detected using highly specific assays, whereas pyrogens require broader biological tests that can capture a wider range of fever-inducing substances [1-3].
Testing Methods Specific to Endotoxins
The primary testing method for endotoxins is the Limulus Amebocyte Lysate (LAL) assay, which uses blood cells from horseshoe crabs to detect gram-negative bacterial endotoxin [1,3]. LAL naturally cross-reacts with 1,3-β-D-glucan through the Factor G pathway, and this is something that should be borne in mind when interpreting results. Whilst blocking buffers are available that inhibit this reaction pathway, the FUJIFILM FDA-licensed Pyrostar™ ES-F product line has been formulated to make it inherently endotoxin-specific, such that you can be confident that the results obtained are due to endotoxin alone.
Recombinant assays that mimic the full LAL cascade (rCR) or specific factors from the lysate (rFC) are also endotoxin-specific and provide an animal-free testing solution [7].
Testing Methods Specific to Pyrogens
Pyrogens (endotoxin and non-endotoxin) can be detected using two different methods [1,3]:
The Rabbit Pyrogen Test (RPT), which assesses fever response in live animals;
The Monocyte Activation Test (MAT), a human cell-based in vitro assay that detects a broad spectrum of pyrogens.
The responses from these assays deliver a ‘bigger picture’ view of the pyrogenicity of the product under test but are unable to provide insight into the relative levels or even the presence of endotoxin and/or specific non-endotoxin pyrogens. Further confirmatory tests are required to determine this.
Pyrogens vs Endotoxins: a Table Summary
What Does “Endotoxin-Free” and “Pyrogen-Free” Mean?
In practice, these terms are marketing and quality claims that are used interchangeably, but there is no universally standardised numerical limit that legally defines “endotoxin-free” or “pyrogen-free” across all product types [1.3]. Most regulations define acceptable maximum endotoxin limits, rather than “zero endotoxin.”
This lack of standardised numerical thresholds means that such labels can be misleading if not properly supported by validated, product-specific testing. Moreover, where products are certified as being “pyrogen free” or “non-pyrogenic” often the only test that has been carried out to verify this property is an endotoxin assay. The overall pyrogenicity of the product has not been evaluated (i.e., tests for non-endotoxin pyrogens have not been included) and therefore the pyrogen-free / non-pyrogenic status is a misnomer.
Find out more about why unquantified endotoxin labels matter:
https://www.cm-dx.com/news-and-resources/what-does-low-endotoxin-really-means
References
[1] United States Pharmacopeial Convention (2020). <85> Bacterial Endotoxins Test. In: United States Pharmacopeia and National Formulary (USP–NF). Rockville, MD: United States Pharmacopeial Convention.
[2] US FDA (2012). Pyrogen and Endotoxins Testing: Questions and Answers. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pyrogen-and-endotoxins-testing-questions-and-answers.
[3] European Directorate for the Quality of Medicines & HealthCare - EDQM (2023). 2.6.14 Bacterial Endotoxins. In: European Pharmacopoeia, 11th ed. Strasbourg: Council of Europe.
[4] Rietschel, ET, et al. (1994). Bacterial endotoxin: Molecular relationships of structure to activity. FASEB Journal, 8(2): 217–225.
[5] Hurley, JC (1995). Endotoxemia: Methods of detection and clinical correlates. Clinical Microbiology Reviews, 8(2): 268–292.
[6] Ding, JL & Ho, B (2001). Endotoxin detection — from limulus amoebocyte lysate to recombinant technologies. FASEB Journal, 15(12): 2347–2365.
[7] United States Pharmacopeial Convention (2025). <86> Bacterial Endotoxins Test using Recombinant Reagents. In: United States Pharmacopeia and National Formulary (USP–NF). Rockville, MD: United States Pharmacopeial Convention.